Background
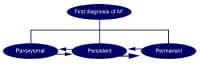
Classification of atrial fibrillation (AF) begins with distinguishing a first detectable episode, irrespective of whether it is symptomatic or self-limited. Published guidelines from an American College of Cardiology (ACC)/American Heart Association (AHA)/European Society of Cardiology (ESC) committee of experts on the treatment of patients with atrial fibrillation recommend classification of AF into the following 3 patterns (also see the image below)[1] :
- Paroxysmal AF – Episodes of AF that terminate spontaneously within 7 days (most episodes last less than 24 hours)
- Persistent AF - Episodes of AF that last more than 7 days and may require either pharmacologic or electrical intervention to terminate
This classification schema pertains to cases that are not related to a reversible cause of AF (eg, thyrotoxicosis, electrolyte abnormalities, acute ethanol intoxication). Atrial fibrillation secondary to acute myocardial infarction, cardiac surgery, pericarditis, pulmonary embolism, or acute pulmonary disease is considered separately because, in these situations, AF is less likely to recur once the precipitating condition has been treated adequately and has resolved.
Paroxysmal AF
Atrial fibrillation is considered to be recurrent when a patient has 2 or more episodes. If recurrent AF terminates spontaneously, it is designated as paroxysmal.
Some patients with paroxysmal AF, typically younger patients, have been found to have distinct electrically active foci within their pulmonary veins. These patients generally have many atrial premature beats noted on Holter monitoring. Isolation or elimination of these foci can lead to elimination of the trigger for paroxysms of AF.
Paroxysmal AF may progress to permanent AF, and aggressive attempts to restore and maintain sinus rhythm may prevent comorbidities associated with AF.
Persistent AF
If recurrent AF is sustained, it is considered persistent, irrespective of whether the arrhythmia is terminated by either pharmacologic therapy or electrical cardioversion.
Persistent AF may be either the first presentation of AF or the result of recurrent episodes of paroxysmal AF. Patients with persistent AF also include those with longstanding AF in whom cardioversion has not been indicated or attempted, often leading to permanent AF.
Patients can also have AF as an arrhythmia secondary to cardiac disease that affects the atria (eg, congestive heart failure, hypertensive heart disease, rheumatic heart disease, coronary artery disease). These patients tend to be older, and AF is more likely to be persistent.
Persistent AF with an uncontrolled, rapid ventricular heart rate response can cause a dilated cardiomyopathy and can lead to electrical remodeling in the atria (atrial cardiomyopathy). Therapy, such as drugs or atrioventricular nodal ablation and permanent pacemaker implantation, to control the ventricular rate can improve left ventricular function and improve quality-of-life scores.
Permanent AF
Permanent AF is recognized as the accepted rhythm, and the main treatment goals are rate control and anticoagulation. While it is possible to reverse the progression from paroxysmal to persistent and to permanent, this task can be challenging.
Lone atrial fibrillation
In addition to the above schema, the term "lone atrial fibrillation" has been used to identify AF in younger patients without structural heart disease, who are at a lower risk for thromboembolism. The definition of lone AF remains controversial, but it generally refers to paroxysmal, persistent, or permanent AF in younger patients (< 60 y) who have normal echocardiographic findings.
Pathophysiology
Atrial fibrillation (AF) shares strong associations with other cardiovascular diseases, such as heart failure, coronary artery disease (CAD), valvular heart disease, diabetes mellitus, and hypertension.[3] These factors have been termed upstream risk factors, but the relationship between comorbid cardiovascular disease and AF is incompletely understood and more complex than this terminology implies. The exact mechanisms by which cardiovascular risk factors predispose to AF are not understood fully but are under intense investigation. Catecholamine excess, hemodynamic stress, atrial ischemia, atrial inflammation, metabolic stress, and neurohumoral cascade activation are all purported to promote AF.
Although the precise mechanisms that cause atrial fibrillation are incompletely understood, AF appears to require both an initiating event and a permissive atrial substrate. Significant recent discoveries have highlighted the importance of focal pulmonary vein triggers, but alternative and nonmutually exclusive mechanisms have also been evaluated. These mechanisms include multiple wavelets, mother waves, fixed or moving rotors, and macro-reentrant circuits. In a given patient, multiple mechanisms may coexist at any given time. The automatic focus theory and the multiple wavelet hypothesis appear to have the best supporting data.
Automatic focus
A focal origin of AF is supported by several experimental models showing that AF persists only in isolated regions of atrial myocardium. This theory has garnered considerable attention, as studies have demonstrated that a focal source of AF can be identified in humans and that isolation of this source can eliminate AF.
The pulmonary veins appear to be the most frequent source of these automatic foci, but other foci have been demonstrated in several areas throughout the atria. Cardiac muscle in the pulmonary veins appears to have active electrical properties that are similar, but not identical, to those of atrial myocytes. Heterogeneity of electrical conduction around the pulmonary veins is theorized to promote reentry and sustained AF. Thus, pulmonary vein automatic triggers may provide the initiating event, and heterogeneity of conduction may provide the sustaining conditions in many patients with AF.
Multiple wavelet
The multiple wavelet hypothesis proposes that fractionation of wave fronts propagating through the atria results in self-perpetuating "daughter wavelets." In this model, the number of wavelets is determined by the refractory period, conduction velocity, and mass of atrial tissue. Increased atrial mass, shortened atrial refractory period, and delayed intra-atrial conduction increase the number of wavelets and promote sustained AF. This model is supported by data from patients with paroxysmal AF demonstrating that widespread distribution of abnormal atrial electrograms predicts progression to persistent AF.[4] Intra-atrial conduction prolongation has also been shown to predict recurrence of AF.[5] Together, these data highlight the importance of atrial structural and electrical remodeling in the maintenance of AF—hence the phrase "atrial fibrillation begets atrial fibrillation.
Etiology
Atrial fibrillation (AF) is strongly associated with the following risk factors:
- Hemodynamic stress
- Atrial ischemia
- Inflammation
- Noncardiovascular respiratory causes
- Alcohol and drug use
- Endocrine disorders
- Neurologic disorders
- Genetic factors
- Advancing age
Hemodynamic stress
Increased intra-atrial pressure results in atrial electrical and structural remodeling and predisposes to AF. The most common causes of increased atrial pressure are mitral or tricuspid valve disease and left ventricular dysfunction. Systemic or pulmonary hypertension also commonly predisposes to atrial pressure overload, and intracardiac tumors or thrombi are rare causes.
Atrial ischemia
Coronary artery disease infrequently leads directly to atrial ischemia and AF. More commonly, severe ventricular ischemia leads to increased intra-atrial pressure and AF.
Inflammation
Myocarditis and pericarditis may be idiopathic or may occur in association with collagen vascular diseases; viral or bacterial infections; or cardiac, esophageal, or thoracic surgery.
Noncardiovascular respiratory causes
Pulmonary embolism, pneumonia, lung cancer, and hypothermia have been associated with AF.
Drug and alcohol use
Stimulants, alcohol, and cocaine can trigger AF. Acute or chronic alcohol use (ie, holiday or Saturday night heart, also known as alcohol-related cardiomyopathy) and illicit drug use (ie, stimulants, methamphetamines, cocaine) have been specifically found to be related to AF.
Endocrine disorders
Hyperthyroidism, diabetes, and pheochromocytoma have been associated with AF.
Neurologic disorders
Intracranial processes such as subarachnoid hemorrhage or stroke can precipitate AF.
Familial AF
A history of parental AF appears to confer increased likelihood of AF (and occasional family pedigrees of AF are associated with defined ion channel abnormalities, especially sodium channels).[6] One cohort study suggests that familial AF is associated with an increased risk of AF. This increase was not lessened by adjustment for genetic variants and other AF risk factors.[7]
Advancing age
AF is strongly age-dependent, affecting 4% of individuals older than 60 years and 8% of persons older than 80 years.
Epidemiology
Atrial fibrillation affects more than 2.2 million persons in the United States. AF is strongly age-dependent, affecting 4% of individuals older than 60 years and 8% of persons older than 80 years. Approximately 25% of individuals aged 40 years and older will develop AF during their lifetime.[8]
The prevalence of AF is 0.1% in persons younger than 55 years, 3.8% in persons 60 years or older, and 10% in persons 80 years or older. With the projected increase in the elderly population in the United States, the prevalence of AF is expected to more than double by the year 2050. AF is uncommon in childhood except after cardiac surgery.[9]
The incidence of AF is significantly higher in men than in women in all age groups. AF appears to be more common in whites than in blacks, with blacks have less than half the age-adjusted risk of developing AF.
In 10-15% of cases of AF, the disease occurs in the absence of comorbidities (lone atrial fibrillation). However, AF is often associated with other cardiovascular diseases, including hypertension; heart failure; diabetes-related heart disease; ischemic heart disease; and valvular, dilated, hypertrophic, restrictive, and congenital cardiomyopathies.[8] The Atherosclerosis Risk in Communities (ARIC) Study suggests reduced kidney function and presence of albuminuria are strongly associated with AF.[10]
The rate of ischemic stroke in patients with nonrheumatic AF averages 5% a year, which is somewhere between 2 and 7 times the rate of stroke in patients without AF. The risk of stroke is not due solely to AF; it increases substantially in the presence of other cardiovascular diseases.[11] The prevalence of stroke in patients younger than 60 years is less than 0.5%; however, in those older than 70 years, the prevalence doubles with each decade.[12] The attributable risk of stroke from AF is estimated to be 1.5% for those aged 50-59 years, and it approaches 30% for those aged 80-89 years.
Prognosis
AF is associated with a 1.5- to 1.9-fold higher risk of death, which is in part due to the strong association between AF and thromboembolic events, according to data from the Framingham heart study.[13]
Medical therapies aimed at rhythm control offered no survival advantage over rate control and anticoagulation, according to the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) trial. The study addressed whether rate control and anticoagulation are sufficient goals for asymptomatic, elderly patients.[14]
Atrial fibrillation (AF) is associated with increased morbidity and mortality, in part due to the risk of thromboembolic disease, particularly stroke, in AF and in part due to its associated risk factors. Studies have shown that individuals in sinus rhythm live longer than individuals with AF. Disruption of normal atrial electromechanical function in AF leads to blood stasis. This, in turn, can lead to development of thrombus, most commonly in the left atrial appendage. Dislodgement or fragmentation of a clot can then lead to embolic phenomena, including stroke.
Development of AF predicts heart failure and is associated with a worse New York Heart Association Heart Failure classification. AF may also worsen heart failure in individuals who are dependent on the atrial component of the cardiac output. Those with hypertensive heart disease and those with valvular heart disease are particularly at high risk for developing heart failure when AF occurs. In addition, AF may cause tachycardia-mediated cardiomyopathy if adequate rate control is not established.
The risk of stroke from AF that lasts longer than 24 hours is a major concern and is usually addressed by prescribing a blood thinner (Coumadin or dabigatran). Prognostic score systems, such as CHAD2, appear to underestimate risk of embolic stroke in patients older than 75 years; thus, some studies recommend treating all patients older than 75 years, unless a compelling contraindication is noted.[15] The CHADS2 score predicts ischemic stroke not only for patients with a history of atrial fibrillation but also for patients without atrial fibrillation who have a history of coronary heart disease.[16] In the latter group, net benefit of prophylactic anticoagulation has yet to be established.
Atrial fibrillation in association with acute myocardial infarction
AF is a common finding in patients presenting with an acute myocardial infarction. A meta-analysis pooled data from 43 studies and more than 278,800 patients.[17] The study found that AF in the setting of acute myocardial infarction was associated with 40% increase in mortality compared to patients in sinus rhythm with acute myocardial infarction. The causes of death were unclear, but may be related to triple anticoagulation therapy with aspirin, clopidogrel, and warfarin, or may be related to hemodynamic consequences associated with the loss of atrial contraction. Whether AF is a complication of myocardial infarction or a marker for myocardial infarction severity is unclear.
Patient Education
A study by van Diepen et al suggests that patients with heart failure or atrial fibrillation have a significantly higher risk of noncardiac postoperative mortality than patients with coronary artery disease; thus, patients and physicians should consider this risk, even if a minor procedure is planned.[18]
For excellent patient education resources, visit eMedicine's Heart Center andStroke Center. Also, see eMedicine's patient education articles Atrial Fibrillation, Heart Rhythm Disorders, Stroke, and Supraventricular Tachycardia.



Tidak ada komentar:
Posting Komentar